Page 1 of 1
Fermenting without yeast
Posted: Thu Mar 26, 2015 8:37 pm
by Drunk-N-Smurf
I really wanted to participate in this conversation from the "campfire whiskey thread" but it wasn't on topic, so.....
contrahead wrote:moosemilk wrote: In a controlled environment with filtered air an no possible contamination of yeast or bacteria, the enzymes alone will not produce alcohol. Please explain why not.
I don't want to be argumentative or drag the conversation away from 'grizz's' corn whiskey topic. I just want to clarify a few points.
- Eduard Buchner won the 1907 Nobel Prize in Chemistry for isolating the enzyme zymase from yeast. He proved that zymase ALONE will catalyze the fermentation of sugar into ethanol and carbon dioxide.
- Even a relatively simple disaccharide sugar like sucrose must first be cleaved into more fundamental sugars like glucose & fructose, by an ENZYME invertase, before normal fermentation (by zyamase produced by yeast) can commence. It just so happens that both invertase and zymase are produced by the yeast we commonly employ, but invertase can be produced from a variety of sources. Even honey bees produce invertase.
- I cannot count the number of wild yeast that may have already resided in my otherwise clean fermentation carboy or which may have been captured from the atmosphere during the few short moments of the mash transfer from stock pot to carboy. However this number of wild yeast seems extremely miniscule compared to a normal inoculation where literally billions of yeast are added at once from an average yeast package. Realizing that yeast cells can reproduce or double every couple of hours (under advantageous conditions in an aerobic environment), this still does not satisfactorily explain to my pleasure, the robust fermentation that I have witnessed - in say 48 hours.
- It wasn't until the mid 19th century that scientific mycological studies began to blossom. By then bakers were beginning to skim and collect froth or barm from the tops of ale vats, German biermiesters were discovering lager yeast and individuals like Louis Pasteur and Emil Christian Hansen were examining yeast under microscopes. Before that time yeast was an unknown. People were utilizing yeast but they didn't know what it was. Moonshiners and distillers, up till, including and after George Washington did not add yeast to their mash.
- I think it wise to be considerate and cautious when adding sugar and some various commercial yeast to a corn whiskey recipe. First of all, if you add a lot of sugar then you’re probably going to get a hot taste that is more like rum than it is whiskey. Secondly, any skilled wine maker will tell you that the choice of yeast strain will have a profound effect upon the flavor profile outcome.
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 8:46 pm
by Drunk-N-Smurf
Ok, my contribution.
Everyone is right. You need yeast to access the enzymes responsible for fermentation, but it is the enzymes that perform the fermentation.
Can you kill yeast and still have the appropriate enzymes? Yes. Under the right conditions.
Edit: is it practical to intentionally do? Not in the slightest.
I found this:
http://chestofbooks.com/food/beverages/ ... RTaTxhOKrU
It's a good read, it described the enzymes responsible for fermentation of sugar, (invertase and zymase) which are present in yeast. PRESENT IN YEAST, NOT TO BE CONFUSED WITH GIVEN OFF OR EXCRETED BY YEAST,.
They are digestive enzymes to yeast the same as enzymes found within our own digestive system.
These enzymes can be harvested, so, it stands to reason that theoretically, under perfect circumstances, wild yeast present on grains could be killed during mashing, but retain active enzymes that could possibly cause fermentation.
A number of observations on the sensitiveness of the chief enzymes of ordinary yeast towards various reagents are recorded by T. Bokorny.1 Thus as regards the invertase, yeast placed in absolute alcohol for several days showed no loss of inverting power when removed. On the other hand, the invertase is destroyed in twenty-four hours by a 1 per cent, solution of caustic soda, though not by a 5 per cent, solution of formaldehyde. Towards acids also it is fairly stable. Fifty per cent, alcohol destroys the zymase activity within twenty-four hours, but 20 per cent, does not. Sulphuric or hydrochloric acid of 1 per cent, strength also destroys the activity within twenty-four hours, as do lactic, acetic, and butyric acids at 5 per cent, strength, though not at 2 per cent. Ammonia solution at a concentration of 0.05 per cent, destroys the zymase activity within forty-eight hours, and formaldehyde at 1 per cent, does so within twenty-four hours. Solutions of neutral salts at concentrations below 10 per cent, are harmless, and in some cases beneficial. As regards maltase activity, 1 per cent, caustic soda solution destroys this in a few hours, but 0 02 and 0.1 per cent, solutions have no appreciable effect in twenty-four hours. Within the same period, 10 per cent. alcohol, 1 per cent, acetic, lactic, or hydrochloric acid, or 0 1 per cent. formaldehyde, all act deleteriously.
"Permanent" yeast. - Zymin or permanent yeast ("acetone yeast".)is a dry preparation obtained by well mixing finely-divided pressed brewers' yeast (500 grams) with acetone (3 litres) for ten minutes to destroy thevitality of the yeast-cells, and then filtering the mass and draining it with the filter-pump. The yeast is then again mixed with acetone (1 litre) for two minutes, filtered, drained, roughly powdered, and well kneaded with ether (250 c.c.) for three minutes, after which it is once more filtered and drained, and then spread on filter paper or porous plates and allowed to dry in the air for an hour. Finally, it is dried for twenty-four hours at 45°.
The product is a nearly white powder in which the yeast-cells are dead, and which therefore cannot grow and reproduce itself. Its cell-walls, however, are intact, and its sugar-fermenting enzyme, zymase, is still active; so that even after keeping for years it will, when ground up and placed in a suitable sugar solution, induce a vigorous alcoholic fermentation.
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 8:50 pm
by moosemilk
You best be ready Cuz you gone done it. May as well have asked where to put a thermometer in a pot still.
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 8:56 pm
by Drunk-N-Smurf
moosemilk wrote:You best be ready Cuz you gone done it. May as well have asked where to put a thermometer in a pot still.
Haha, yeah, but better in its own thread than in someone else's help me thread

Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 9:26 pm
by moosemilk
It is interesting though, imagine...powdered alcohol conversion. I wonder what kind of esters if any, would be produced by this?
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 9:32 pm
by Drunk-N-Smurf
moosemilk wrote:It is interesting though, imagine...powdered alcohol conversion. I wonder what kind of esters if any, would be produced by this?
I think with the right set of isolated enzymes, could probably pull off completely pure etoh and co2
The trick would be cost effectively extracting and isolating those enzymes.
Simplistic look:
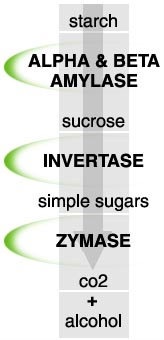
- image.jpg (15.46 KiB) Viewed 4745 times
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 9:41 pm
by ranger_ric
I am STILL laughin at moosemilk's reply. G D that was Frickin Hilarious !!! (I even saw it coming but couldn't help but ROFL.!!!)
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 9:45 pm
by Drunk-N-Smurf
For split second, I debated making that can pic my profile pic...seems I have a stockpile I keep opening :p
Re: Fermenting without yeast
Posted: Thu Mar 26, 2015 11:19 pm
by Drunk-N-Smurf
Well, now it's got me thinking.
A quick google search of "yeast extract" resulted in products like marmite, and Vegemite, and straight up yeast extract (used as a sub for msg)
Reading a bit about the process used, it sounds like it's essentially all the stuff that makes yeast yeast, without the living cell wall.
So I took a glancing through the forums....people have had some good success using these products as "nutrients" in their washes, reporting better, faster ferments..
Edit: Evidenced here, also not the nutritional yeast and autolysed yeast (dead yeast essentially) had a similar effect of speeding up the fermentation. In this experiment the other yeast products were being used as nutrients for the yeast, but given the theory, it could be theorized that they were actually acting WITH the pitched yeast to speed the ferment.
So, I propose a test. Start a basic 5gallon sugar wash, instead of yeast, throw in some "yeast extract" product. How much would be the question, maybe 1tsp/gal to start? If my theory is correct, it should ferment itself out...
Damnit, now I have to try to find marmite within walking distance.
Science bitches.
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 4:46 am
by scuba stiller
http://www.amazon.com/s?ie=UTF8&page=1& ... %3AMarmite" onclick="window.open(this.href);return false;" rel="nofollow
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 4:49 am
by heartcut
Anyone up for a concurrent experiment on reproduction without sex?
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 9:28 am
by Drunk-N-Smurf
heartcut wrote:Anyone up for a concurrent experiment on reproduction without sex?
Haha, women have been doing THAT for years.
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 9:43 am
by moosemilk
Drunk-N-Smurf wrote:
Damnit, now I have to try to find marmite within walking distance.
Science bitches.
Marmite is carried in most grocery stores here In Canada and found in the bakers section by the yeast (I find that silly, because it's a spread...should be with jams and jellies and such). Vegemite I find at my local bulk store. It's a bit pricier that way, but I like it enough. Mmmm, toast with butter and vegemite.
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 9:45 am
by Drunk-N-Smurf
moosemilk wrote:Drunk-N-Smurf wrote:
Damnit, now I have to try to find marmite within walking distance.
Science bitches.
Marmite is carried in most grocery stores here In Canada and found in the bakers section by the yeast (I find that silly, because it's a spread...should be with jams and jellies and such). Vegemite I find at my local bulk store. It's a bit pricier that way, but I like it enough. Mmmm, toast with butter and vegemite.
Yeah, my keyword is "walking distance", I'm not taking a bus for a jar of marmite lol. Though my curiosity may force me to.
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 11:18 am
by moosemilk
You haven't tried it? It's pretty potent. Very salty. Kinda like a malty beef bullion cube. Use it sparingly. And some sharp cheddar on top makes it awesome.
I thought of trying it as a nutrient, but the sodium level is through the roof on it!
Re: Fermenting without yeast
Posted: Fri Mar 27, 2015 12:48 pm
by Drunk-N-Smurf
moosemilk wrote:You haven't tried it? It's pretty potent. Very salty. Kinda like a malty beef bullion cube. Use it sparingly. And some sharp cheddar on top makes it awesome.
I thought of trying it as a nutrient, but the sodium level is through the roof on it!
No never tried it, I know a few Brits that eat it all the time but never built the nerve to try myself.
Sodium content is likely so high due to the salt the use to cause the yeast cell to die. But I think hookline uses it regularily as a nutrient.
Re: Fermenting without yeast
Posted: Sat Mar 28, 2015 12:15 am
by cb_j
It's expensive. A can of tomato paste vs a jar of Vegemite.
But in the express purpose relating to this post, maybe not.
It would be un-Australian of me if I didn't have a Jar if it in stock, regardless of cost.
Re: Fermenting without yeast
Posted: Sat Mar 28, 2015 3:34 am
by thecroweater
Vegemite will work as a nutrient not as a substitute for yeast on another note Vegemite spread thin on rye bread with parmesan cheese is the bomb
Re: Fermenting without yeast
Posted: Sun Mar 29, 2015 4:18 am
by Matt86
Drunk-N-Smurf wrote:Well, now it's got me thinking.
A quick google search of "yeast extract" resulted in products like marmite, and Vegemite, and straight up yeast extract (used as a sub for msg)
Reading a bit about the process used, it sounds like it's essentially all the stuff that makes yeast yeast, without the living cell wall.
So I took a glancing through the forums....people have had some good success using these products as "nutrients" in their washes, reporting better, faster ferments..
Edit: Evidenced here, also not the nutritional yeast and autolysed yeast (dead yeast essentially) had a similar effect of speeding up the fermentation. In this experiment the other yeast products were being used as nutrients for the yeast, but given the theory, it could be theorized that they were actually acting WITH the pitched yeast to speed the ferment.
Yeah, I doubt there is any "zymase" activity remaining after the processing those products go through. However any improvement in yeast health via nutrient addition is going to improve fermentation rates. I use yeast extract as a nutrient in my sugar washes - it has a long history of being used in culturing microorganisms.
Can I get a link to where you got that table? Looks like someone has been doing interesting work. EDIT -Found it!
Drunk-N-Smurf wrote:
So, I propose a test. Start a basic 5gallon sugar wash, instead of yeast, throw in some "yeast extract" product. How much would be the question, maybe 1tsp/gal to start? If my theory is correct, it should ferment itself out....
If you get fermentation its far more likely to be a bacterial or yeast containment.
Re: Fermenting without yeast
Posted: Sun Mar 29, 2015 5:49 am
by masonsjax
The way I understand it (I am not a scientist) is that the enzymes catalyze the fermentation, they don't actually do the fermenting. They are responsible for dividing complex sugars into short chain sugars which makes them available for the yeast to convert into ethanol and other compounds. My guess is that adding just a bunch of enzymes without yeast would just change sweet wort to a slightly different sweet wort, provided the temperatures are within the enzymes active range, otherwise nothing would happen.
Re: Fermenting without yeast
Posted: Sun Mar 29, 2015 9:14 am
by Drunk-N-Smurf
Enzymes catalyze a chemical reaction. The conversion of alcohol is just that, a chemical reaction. Industrial ethanol is made using chemicals to complete the reaction, not yeast, but the method is not recommended do to the residual potential of the extremely toxic chemicals that remain once the reaction is complete.
Yeast perform this chemical reaction inside them using various enzymes to perform the chemical reactions. The Evidence was already proven, however, if your going to harvest the enzymes from yeast just for fermentation, you might as well just use the yeast to begin with.
But inquiring minds will always do the least sensible things to find answers to their queries

Re: Fermenting without yeast
Posted: Mon Mar 30, 2015 10:18 am
by Ferment_It
Drunk-N-Smurf wrote:. Industrial ethanol is made using chemicals to complete the reaction, not yeast, but the method is not recommended do to the residual potential of the extremely toxic chemicals that remain once the reaction I....

LOL [GRINNING FACE WITH SMILING EYES]!
You are joking right?!
Re: Fermenting without yeast
Posted: Mon Mar 30, 2015 10:47 am
by Drunk-N-Smurf
Ferment_It wrote:Drunk-N-Smurf wrote:. Industrial ethanol is made using chemicals to complete the reaction, not yeast, but the method is not recommended do to the residual potential of the extremely toxic chemicals that remain once the reaction I....

LOL [GRINNING FACE WITH SMILING EYES]!
You are joking right?!
Nope.
http://www.essentialchemicalindustry.or ... hanol.html
Ethanol is manufactured by the direct catalytic hydration of ethene in the presence of steam, using phosphoric acid adsorbed on the surface of a solid (silica)
as a catalyst in a fixed bed reactor. The reaction is reversible and exothermic:
From the equilibrium equation, it can be seen that conversion of the feedstock to ethanol is favoured by low temperature, high pressure and high steam concentration.
To achieve acceptable reaction rates, a temperature of ca 500 K is used in the presence of the catalyst. Increasing the pressure pushes the reaction to the product side but also causes polymerization of the ethene. Higher pressures also mean increased capital and operating costs. In practice, the process is generally operated under a pressure of 60-70 atm.
Re: Fermenting without yeast
Posted: Mon Mar 30, 2015 11:14 am
by Ferment_It
Well shit. Sorry 'smurf. Now I feel like an ass... And i Googled it before I posted.
Change of search terms brought me to FDA.gov- FDA allows synthetic etoh in medicinal and flavoring and food ingredients.
Re: Fermenting without yeast
Posted: Mon Mar 30, 2015 11:19 am
by Drunk-N-Smurf
Ferment_It wrote:Well shit. Sorry 'smurf. Now I feel like an ass... And i Googled it before I posted.
Change of search terms brought me to FDA.gov- FDA allows synthetic etoh in medicinal and flavoring and food ingredients.
No need to feel like an ass. I spend 6-8 hrs a night after the wife goes to bed on a normal day, and since I've been unemployed spent pretty much 18-20 hrs a day bored stupid googling and researching anything and everything.
Pretty much anything that comes into debate around here, I'll spend literally hours on Google looking for information relating to the argument. Sometimes I learn something new which is always fun.
Re: Fermenting without yeast
Posted: Sun Apr 05, 2015 7:41 pm
by Killrb13
Well technically theres Zymomonas mobilis and its not yeast.

Anyone ever do any research on that bacteria? Kinda interesting really..
Re: Fermenting without yeast
Posted: Wed Apr 08, 2015 6:36 am
by contrahead
Killrb13 wrote:Well technically theres Zymomonas mobilis and its not yeast.

Anyone ever do any research on that bacteria? Kinda interesting really..
Thanks for your Zymomonas mobilis lead. Fermentation by bacteria is a nebulous unknown to me and I need to do plenty more research on the subject. Didn’t even know this thread was over here until today – as I’ve been preoccupied by other activities for the last couple of months.
Re: Fermenting without yeast
Posted: Mon Apr 20, 2015 7:16 pm
by MDH
Drunk-N-Smurf wrote:
http://www.essentialchemicalindustry.or ... hanol.html
Ethanol is manufactured by the direct catalytic hydration of ethene in the presence of steam, using phosphoric acid adsorbed on the surface of a solid (silica)
as a catalyst in a fixed bed reactor. The reaction is reversible and exothermic:
From the equilibrium equation, it can be seen that conversion of the feedstock to ethanol is favoured by low temperature, high pressure and high steam concentration.
To achieve acceptable reaction rates, a temperature of ca 500 K is used in the presence of the catalyst. Increasing the pressure pushes the reaction to the product side but also causes polymerization of the ethene. Higher pressures also mean increased capital and operating costs. In practice, the process is generally operated under a pressure of 60-70 atm.
It's perfectly possible to make safe drinking ethanol with this process. The issue is practicality on a small scale, not food safety.