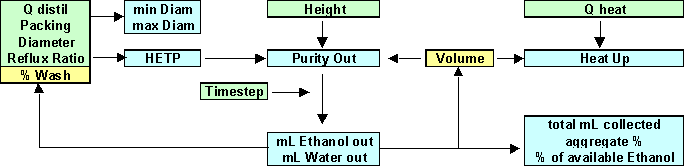

Knowing the about the wash & column, we can calculate things like recommended min & max column diameters, the purity we expect off the top column, and how long it will take to heat up to temperature. Then, we can look at the column performance over time - how much we expect to collect from it, and how this changes (as does the column performance) over the course of the run.
I've collated all the required Physical constants, etc for water & ethanol on a data page
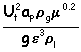 (call this "y") vs
(call this "y") vs
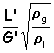 (call this "x")
(call this "x")
Plotting the flooding line, I found : y = =1.4427 x2 -1.0271 x + 0.2312 where
Through the top section we have
Based on the composition of the wash, and the power input (Q distill), you can estimate the amount of vapour you are generating (V in kg/s)
Q distill = (V * %ethanol * Hvap ethanol) + (V * %water * Hvap water)
Then G' = V / column area ( pD2)
The amount of liquid (L) being refluxed will depend on the Reflux ratio; since D=V-L, and the Reflux ratio is L/D, then
![]()
So then L' = L / column area
(hmmm.. this is where I do something a little bit dodgy... we are estimating the amount of vapour being generated off the wash. This will be true for the bottom of the column, but may not be quite the same as the vapour flowrate at the very top - some of the water will have been removed from it, and it will have picked up some more alcohol vapour on the way ... So what I do is a wee mass balance based on the expected purity at the top. This can get you into a catch 22 - because you don't know this until you can work out the HETP for the packing, but you need this value to calculate the HETP.. so guess a final %, run through the calcs, see what the top % actually is, and adjust from there & recalculate if necessary).
So... calculate out x =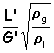 , solve for y, then
work out Ut
, solve for y, then
work out Ut
Since Ut = G' / area of column, and knowing area = p D2 you can solve for D Flood
This is the flooding diameter - eg smaller than this will cause the vapour flowrate to be too fast to allow the liquid to drain down past it. It is the absolute minimum diameter.
So the column needs to be a little wider. Standard recommendations are to use approx 65% the flooding velocity, so scale up the diameter we should use by
![]()
Likewise, if the column is too wide, there won't be enough interaction between the liquid and the vapour. I can't find any recommendations for this, but I'm guessing about 25% would be about it, so :
![]()
Now the flowrates of Liquid & Gas will be different at different heights of the column... so once you've been through the whole exercise, redo this calculation a couple of times, at a couple of different heights (flowrates). You find out that the differences aren't really worth worrying about.
Calculate the effective area (aw) using :
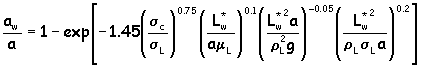
where:
| Critical surface tension | |
| Material | sc [mN/m] |
| Ceramic | 61 |
| Metal (steel) | 75 |
| Plastic (polyethylene) | 33 |
| Carbon | 56 |
Then calculate the liquid and gas mass transfer coefficients (kL and kG) using :
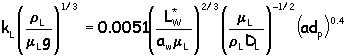
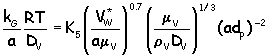
where:
From these you can then calculate the film transfer heights:
![]()
![]()
where:
From these you can then estimate the height of the overall gas-phase transfer unit:
![]()
Now... for a section of the packed column in which the operating and equilibrium lines can be considered straight (hey- that's almost us !), theoretical stages can be converted to numbers of transfer units by :
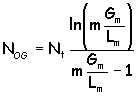
and then
![]()
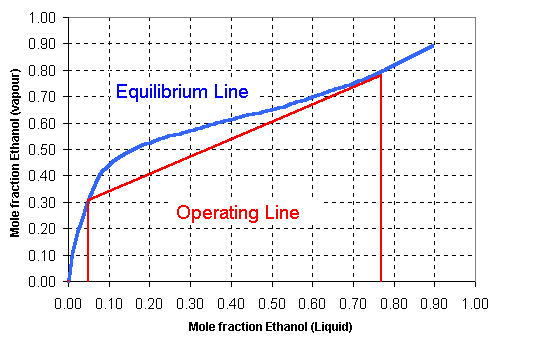
Estimate the purity at the top of the column by stepping off the correct number of theoretical stages on the Equilibrium diagram
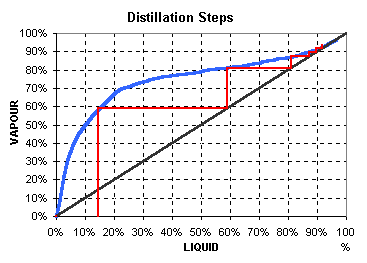
y = -31.065 x6 + 116.08 x5 - 169.95 x4 + 123.99 x3 - 47.195 x2 + 9.1398 x
So... pick a suitable time-step, then calculate the volume removed (flowrate * time). You know the purity of it, so you can then update the total removed, the aggregate purity, and the purity and volume of the wash left in the pot.
For each time-step you can then go back to the start and redo it all again (HETP etc) if you really want to....but its not really worth the effort, as these don't change much.
Just keep a good track of the various units you're using, cos there's a bit of switching between moles, kgs, and grams along the way (let alone dabbling in Imperial units...).
Tony Ackland 3 June 2000