Maturation fixed acidity: Difference between revisions
Jump to navigation
Jump to search
Uncle Jesse (talk | contribs) Initial page creation |
Uncle Jesse (talk | contribs) No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[file:Maturation_fixed_acidity.gif|thumb|250px|right|Fixed acidity changes during maturation]] | |||
The acidity of the [[ | The acidity of the [[spirit]] increase rapidly at first, and then levels off. For this plot, the acidity is measured in grams of acetic acid per 100 liters. As a comparision, white vinegar, which is 5% acetic acid would have an acidity rating of around 4000 on this chart. Or stated another way, an ounce of vinegar added to a four gallon bucket of water would have about the same acidity as [[whisky]]. | ||
From ''Changes in Whisky While [[Maturation|Maturing]]'' by A.J. Liebmann and Maurice Rosenblatt in the September 1943 issue of Industrial and Engineering Chemistry. | |||
From ''Changes in Whisky While Maturing'' by A.J. Liebmann and Maurice Rosenblatt in the September 1943 issue of Industrial and Engineering Chemistry. | |||
[[Category:Maturation]] | [[Category:Maturation]] | ||
Latest revision as of 23:14, 15 December 2022
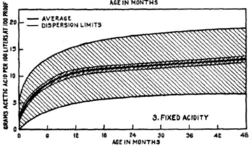
The acidity of the spirit increase rapidly at first, and then levels off. For this plot, the acidity is measured in grams of acetic acid per 100 liters. As a comparision, white vinegar, which is 5% acetic acid would have an acidity rating of around 4000 on this chart. Or stated another way, an ounce of vinegar added to a four gallon bucket of water would have about the same acidity as whisky.
From Changes in Whisky While Maturing by A.J. Liebmann and Maurice Rosenblatt in the September 1943 issue of Industrial and Engineering Chemistry.