Butanol
Jump to navigation
Jump to search
Butanol is a higher alcohol with a 4 carbon atoms and a general formula of C4H10O. There are 4 different isomeric structures for butanol:
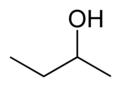
|

|
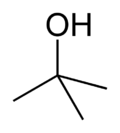
| |
| n-butanol | sec-butanol | isobutanol | tert-butanol |
These butanol isomers, due to their different structures, have somewhat different melting and boiling points. All are moderately miscible in water and used as a base for perfumes. For the purposes of distillation, butanol is considered to be a fusel oil. Like most alcohols, they are poisonous.