Amyl alcohol
There are eight isomers of amyl alcohol (C5,H11OH):
Amyl alcohol isomers Common name Structure Type IUPAC name Boiling point (°C)[1] normal amyl alcohol 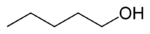
primary Pentan-1-ol 138.5 isobutyl carbinol
or isoamyl alcohol
or isopentyl alcohol

primary 3-Methylbutan-1-ol 131.2 active amyl alcohol 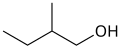
primary 2-Methylbutan-1-ol 128.7 tertiary butyl carbinol
or neopentyl alcohol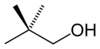
primary 2,2-Dimethylpropan-1-ol 113.1 3-Pentanol 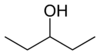
secondary Pentan-3-ol 115.3 methyl (n) propyl carbinol 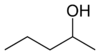
secondary Pentan-2-ol 118.8 methyl isopropyl carbinol 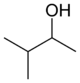
secondary 3-Methylbutan-2-ol 113.6 dimethyl ethyl carbinol
or tertiary amyl alcohol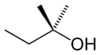
tertiary 2-Methylbutan-2-ol 102
Three of these alcohols, active amyl alcohol, methyl (n) propyl carbinol, and methyl isopropyl carbinol, contain an asymmetric carbon atom and can consequently each exist in two optically active, and one optically inactive form.
The most important is isobutyl carbinol, this being the chief constituent of fermentation amyl alcohol, and consequently a constituent of fusel oils. It may be separated from fusel oil by shaking with strong brine solution, separating the oily layer from the brine layer and distilling it, the portion boiling between 125°C and 140°C. being collected. For further purification it may be shaken with hot lime water, the oily layer separated, dried with calcium chloride and fractionated, the fraction boiling between 128°C and 132°C only being collected. It may be synthetically prepared from isobutyl alcohol by conversion into isovalerylaldehyde, which is subsequently reduced to isobutyl carbinol by means of sodium amalgam.
It is a colorless liquid of density 0.8247 g/cm³ (0°C), boiling at 131.6°C, slightly soluble in water, easily soluble in alcohol, ether, chloroform and benzene. It possesses a characteristic strong smell and a sharp burning taste. When perfectly pure, it is not a poison, although the impure product is. On passing its vapour through a red-hot tube, it undergoes decomposition with production of acetylene, ethylene, propylene, etc. It is oxidized by chromic acid mixture to isovalerylaldehyde; and it forms crystalline addition compounds with calcium and stannic chlorides.
The other amyl alcohols may be obtained synthetically. Of these, tertiary butyl carbinol has been the most difficult to obtain, its synthesis having only been accomplished in 1891, by L. Tissier (Comptes Rendus, 1891, 112, p. 1065) by the reduction of a mixture of trimethyl acetic acid and trimethylacetyl chloride with sodium amalgam. It is a solid which melts at 48 to 50°C and boils at 112.3°C.
References
- ↑ Calculated boiling points from ChemSpider.