Distillation Theory: Difference between revisions
Uncle Jesse (talk | contribs) (That's about enough for tonight.) |
Uncle Jesse (talk | contribs) No edit summary |
||
| Line 140: | Line 140: | ||
George : From what I have read, a general rule of thumb is; up to 4" you would be better off with scrubbers, anything bigger than 4" and scrubber will tend to channel, from 4" up to 8" you would do well with packing like pall rings and the like, from 10" and up you would do well to use plates. However I have seen plans for a 4" still with bubble cap plates and they claim to be the most efficient still made. Their claim not mine. To make the 10" bubble cap plate type still work you would need somewhere around a 10 hp boiler or around 350,000 btu input. It would also produce up to or around 30 gallon of ethanol per hou using a 10% wash. I have gleaned this information from a lot of different sources and complyed it myself, none of it is to be considered absolute. Their has been some writings about using some sort of perforated plate with the packing on the 6 to 8 inch stills to help even out the vapor flow. | George : From what I have read, a general rule of thumb is; up to 4" you would be better off with scrubbers, anything bigger than 4" and scrubber will tend to channel, from 4" up to 8" you would do well with packing like pall rings and the like, from 10" and up you would do well to use plates. However I have seen plans for a 4" still with bubble cap plates and they claim to be the most efficient still made. Their claim not mine. To make the 10" bubble cap plate type still work you would need somewhere around a 10 hp boiler or around 350,000 btu input. It would also produce up to or around 30 gallon of ethanol per hou using a 10% wash. I have gleaned this information from a lot of different sources and complyed it myself, none of it is to be considered absolute. Their has been some writings about using some sort of perforated plate with the packing on the 6 to 8 inch stills to help even out the vapor flow. | ||
On spacing I read once, and do not remember where that the spacing should be double the diameter as a rule of thumb. But the heat input, the quanity output, and the wash percent all effect this so it is hard to say. Their is no set rule to follow. Perforated plates require a lot of drilling and the bubble caps are hard to construct. Anything over a 6 to 8 inch would require quite the effort to bring up to speed. Unless you have a cheap source of heat, a motor of some type that runs constancely , the expense of bringing one of the bigger one up to steam would be very high. | |||
One other thing that effects the plate type stills is wheather or not you are going to filter out the solids in your wash. If you are not then your plates would have to be designed to be self cleaning. If you do then the solids need to be compressed to get as much alcohol recovered as possible. | |||
Gaw : Using the photos ... of a bubble type plate still in Holland I built a four inch eight plate still which seems to work quite well on top of a six gallon electric water heater with benefit of a thermostat which I added. To further the experiment of continuous distilling I added a thirty gallon pot with a connection three plates above the smaller unit and after the complete system reaches operating temps the unit seems to function quite well at 94-95 per cent. I used ss plates which I found in a salvage yard and soldered the bubblers into separate units which I then bolted together, believe it or not, with ss bolts and neoprene gaskets on each end of the four inch pieces. | Gaw : Using the photos ... of a bubble type plate still in Holland I built a four inch eight plate still which seems to work quite well on top of a six gallon electric water heater with benefit of a thermostat which I added. To further the experiment of continuous distilling I added a thirty gallon pot with a connection three plates above the smaller unit and after the complete system reaches operating temps the unit seems to function quite well at 94-95 per cent. I used ss plates which I found in a salvage yard and soldered the bubblers into separate units which I then bolted together, believe it or not, with ss bolts and neoprene gaskets on each end of the four inch pieces. | ||
| Line 149: | Line 149: | ||
I think the best solution for an ethanol distillation would be a packing of copper rings. These should not be too difficult to manufacture. Winding a copperwire on a thin rod with an electric drill and cutting the created spring to rings shouldn't be too difficult. | I think the best solution for an ethanol distillation would be a packing of copper rings. These should not be too difficult to manufacture. Winding a copperwire on a thin rod with an electric drill and cutting the created spring to rings shouldn't be too difficult. | ||
[[Category:How-To]] | [[Category:How-To]] | ||
[[Category:Glossary]] | [[Category:Glossary]] | ||
Revision as of 23:52, 13 November 2022
Summary of Distillation Theory
When you heat up a mixture of liquids, the more volatile components will tend to come off first. There is a bit of overlap (so it is never pure), but generally we can separate the ethanol from the water and other impurities present. The more alcohol in the liquid, the more alcohol will be in the vapor, so multiple distillations allow us to increase the strength & purity right up to 97.2%
The concept of [distillation] is really quite simple.
Mike Nixon has compiled an excellent pdf "Distillation - How it Works" explaining all this a lot clearer than what i have below. Right down to plate theory for heat exchangers, without making it complicated. Either e-mail Mike for it, or download it from me here (95 Kb, 16 Nov 99). A more detailed explanation is in his new book "The Compleat Distiller" at http://www.amphora-society.com.
Another explanation, geared towards distilling ethanol from agricultural products, is Purdue University's [1] note "Alcohol distillation : basic principles, equipment, performance relationships, and safety".
The University of Akron has a slide show covering the basics too. See also : "The Brewery's" Technical Library for articles on brewing related topics (extremely comprehensive) and AllTech's company homepage has much good literature.
Here's a somewhat simplified explanation ....(thanks to Mike)
When you have a mixture of liquids, each with its own boiling point when pure, then the boiling point of the mix will lie somewhere in the middle, and this will depend on the relative concentrations of each liquid. Pure water boils at 100 °C, and pure ethanol boils at 78.5 °C, but a mixture of water and ethanol will boil at some point in between. The major point about distillation is that when a mixture like that boils, then the vapor given off is richer in the most volatile component, and when that vapor condenses then the resulting liquid has a lower boiling point than the mix it came from. By repeating this boiling and recondensation process up a column, using packing to hold the condensed liquid at each stage, you can separate the components more and more.
So if you have a mixture of liquids each with a different boiling point, then you heat the mixture, it will heat up until the new intermediate boiling point is reached. When you first start a distilling run, the packing in the column will be at room temperature, so vapor given off by the boiler condenses on the first cool packing it reaches. In condensing, the vapor gives up a lot of heat, and this warms that packing until the liquid on it boils again. However, this liquid is richer in volatiles than the mix in the boiler, so its boiling point is lower. When it does boil again, from the heat given off by more condensing vapor, what you get is even richer in those most volatile components. This process of boiling and condensing continues up the column and, because the condensed liquid is always getting richer in volatiles, the temperature gradually falls the higher you go. The temperature at any point is governed solely by the boiling point of that liquid mix, and any attempt to interfere with that process will disrupt the separation that Nature is carrying out automatically.
In contrast, the boiling point of the mix left in the boiler will very slowly start to rise as it is left with less and less of the most volatile components.
If you started with a mixture (fermented wash) that is mostly water & ethanol, with trace amounts of methanol, propanol, etc. then the net result will be that the most volatile components will tend to rise in greater quantity up the column than their less volatile cousins, and will be found in greatest concentration at the top. This would mean that methanol, the most volatile of the lot, will win the race and you will able to collect it and set it aside. This continues until you have collected all of the "heads" (components that are more volatile than ethanol), and you can then collect just ethanol with a trace of water. You cannot get rid of that small amount of water, as once you reach a mix of 97.2% ethanol/water, with a boiling point of 78.2 °C, then you have reached a stable mix that no amount of re-boiling and re-condensation can change (at normal atmospheric pressure).
Once you have collected the main bulk of ethanol, then the components that are less volatile than ethanol, such as propanol and the bigger organic molecules, will start to reach the top, and you will have arrived at the stage called the "tails". These "tails" may be recycled in the next batch you do, for they still contain a lot of ethanol, or a proportion may be retained as they contain many of the compounds that give a spirit a distinctive flavor, like [whiskey] or [rum].
Note that you are not changing any part of your original brew - you're not "making" the alcohol, or converting it to something else or nasty. All you are doing is concentrating off the original brew into its various parts. There is no more methanol after you finish than what you started with. What does happen though, is that because most of the methanol comes off at once (first up), it is highly concentrated, and can damage you. You definitely don't want to be sampling the first portion of distillate that you collect. But once you have thrown away this part, you have guaranteed that the remaining distillate is safe enough to partake of.
Distillate Strength
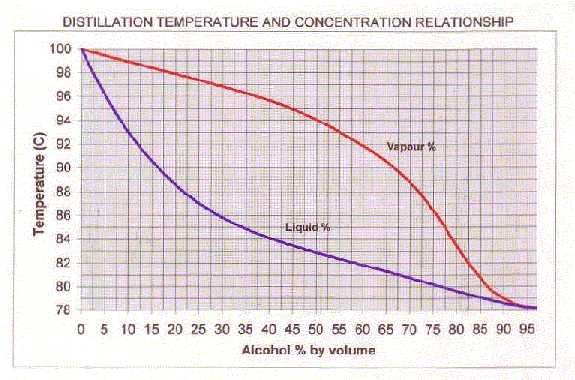
You can use the graph below (thanks to Grant D) to relate a liquid's % alcohol and its boiling point. It also shows the % alcohol of the condensed vapor. (The data for the graph (and heaps of other stuff) is on my Calculation page if you're interested in drawing it yourself.)
For example: A liquid of 10% alcohol will boil at 93 degrees (i.e. the point on the blue line directly above 10 on the [[Alc by Volume]|ABV] axis). If you head horizontally from the 93 degree point until you hit the red line, then drop down to the alcohol axis, it strikes 55%.
So, for a simple still, a 10% alcohol wash will boil at (initially) 93 degrees and the vapor, once condensed, will contain 55% alcohol by volume. Likewise, redistilling a 40% spirit should result in a brew around 80%.
Alcohol liquid - vapor curves
At % alcohol (liquid) the vapor will be % alcohol
Note that only 97.2% ethanol can be obtained by regular distillation of alcohol & water. Absolute ethanol (100%) is made by distilling with benzene which is poisonous. An azeotrope mixture of benzene, alcohol & water distills at 149F (65C) and removes the last few percent of water by vacuum distillation or by chemical means, e.g. using drying agents like molecular sieves with holes of 3 Angstrom or three ten billionths or a meter. This separates water which has a diameter of 2.5 Angstrom from ethanol which has a diameter of 4.5 Angstroms. Update: Phil advises me that most major commercial distilleries in Australia use Cyclohexane rather than Benzene.
Reflux Still Design
To increase the purity of the alcohol, and hence reduce the amount of "off-flavours" in it, you need to use a taller column, packed with something which has a large surface area (scrubbers are best), and have some of the vapour condensing and being returned back down over the packing as a liquid (reflux).
For a certain height of packing (called the HETP), the purity will improve - roughly 1x = approx 85% purity, 3x = 90%, 5x = 93%, and 7-9xfor 95%+. Just make it as high as what you want pure. For scrubbers the HETP is about 10cm (4 inches), whereas it is 24-38 cm (10-16 inches) for raschig rings or marbles.
See my Equations used during Reflux Distillation Calculations if you want to get into the detail of this.
Purity is improved during distillation by allowing the rising vapor to mingle with some liquid at a slightly cooler temperature. In doing so, some of the water rich vapor will condense, supplying a bit of energy to allow some alcohol rich vapor to form from the liquid , and join the existing vapor. Each time this "mingling" is sufficient to reach equilibrium, the purity takes a "step" on the graph below:
Purity: Ethanol/Water Distillation Equalibrium Demonstration
Thanks to Chris Noonan for helping do this Applet.
This graph is of the "ethanol-water equilibrium" eg a liquid of 15% alcohol will be in equilibrium with a vapor at 65% alcohol. If this 65% vapor is then cooled to form a liquid (it will remain at 65%), the new liquid would then be at equilibrium with a 84% vapor, and so on. If you have a pot still, just set the plates to one.
You can see that due to the shape of the curve, most of the gains are early on; to get to the really high % purity, you need to take lots of steps later on. There is no way around this. If you want high purity, you have to work hard for it. Also note (particularly for inefficient columns with the equivalent of only 1-2 plates) that the starting % can also affect the final % achieved - hence a good idea to use the better yeasts.
Each of these "steps" represents an "ideal plate" where enough mingling of liquid & vapor allows them to come to equilibrium. If you don't allow enough mingling (equilibrium), then you won't achieve a full step, but end up a little shy of the target. You get the first step free - its the boiler/pot. Basically, off a 10% wash
1 = 53%
2 = 80%
3 = 87%
4 = 90%
5 = 92%
6 = 92.6%
7 = 93.3%
8 = 93.8%
9 = 94.2%
10 = 94.4%
One way of doing these steps is to do many single distillations, collect the vapour that comes off, condense it, clean out the still, and run it through the still again. This why pot stillers do double & triple distillations to get into the 80+ % range. But a Reflux column allows this to happen continuously; if given enough surface area to equalibriate on, the vapor can have gone through multiple distillations by the time it gets to the top of the column.
For each plate to work, it has to be at a particular temperature, slightly cooler than the one below, and warmer than the one above. Only then will it achieve its equilibrium and an increase in the alcohol purity. The differences are really fine too — its all happening only between 78.1 C and 82.2 C — quite a tight band to walk between.
Mike Nixon explains in a bit more detail ...
The process of separation depends on two facts:
1) when a vapor condenses then the resulting liquid has the same composition as the vapor, and the temperature at which this occurs is the same as the boiling point of that mixture. The boiling point lowers as the proportion of volatiles increases, so the temperature as you go up a column naturally decreases. One sticking point is that many think that a vapor only condenses when it encounters a surface that is cooler than the boiling point, but this is not so. Condensation occurs when there is a path for the latent heat of vaporization/condensation to be removed from the vapor, and the resulting liquid will remain at its boiling point if no further heat is removed.
2) when this liquid re-evaporates then the resulting vapor is richer in the most volatile components.
The packing is there simply to hold intermediate distillate in place so it can be bathed in hot, rising vapor and allow this second process to occur. As volatiles are further extracted from the intermediate distillate, the boiling point of what remains increases and the depleted liquid builds up, eventually dripping down the packing to a hotter level where it can again be stripped of more volatiles.
A cooling tube placed near the bottom of a column simply interrupts this natural progression and serves no useful purpose in the separation process. In contrast, the top cooling tube IS useful as it helps to return some of the vapor arriving at the top of the column to the packing, where it has a further chance of being stripped more thoroughly. This is what a condenser placed on top of a compound column does, but with more efficiency.
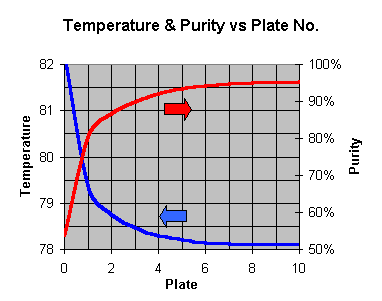
Temp and Purity Vs Number of Plates if Column Stills
This is where the various designs that have cooling tubes running through their columns at all different heights (eg Labmaster) come adrift — they don't allow the required sequence of temperatures to develop fully, and thus won't work at their full potential. They also don't allow all the refluxed liquid to do its job over the packing — the less liquid/vapor contact the poorer the "polishing" of the vapor will be.
Heat & Mass Balance calculations in Reflux Stills
This is why you should also (see my interactive Heat & Mass Balance page to play with these and see it for yourself):
- Insulate the column well (don't want breezes causing additional cooling out of sequence),
- Let the column run at total reflux for a while (to allow the packings to heat up to their equilibrium temperature). This is also important so that the methanol is given a chance to all work its way to the top of the column, so that it will all come off in the first off-take,
- Only have condensors for reflux above the packing, and
- Use a stable/continuous heat source (you don't want it switching off & on all the time causing surges of vapour going up the column then periods of nothing; it has to be a steady continuous flow of vapour & liquid)
So you can easily work out what is required to get a particular % purity; just look up the number of ideal plates needed, eg 2 plates = 87%, 3=90%, 4=92% and so on. Remember you get the first one free - its the pot.
A pot still is the equivalent of a single plate; if it has "thumpers" attached to it, each of these can act as an extra plate.
Why call them plates ? In large distillation columns, they are exactly that; large metal plates or trays, which the liquid flows over, and the gas bubbles up through holes in them. However they are quite tricky to design & build, and not really suited for small column diameters (say less than 1 ft diameter) - they're just too fiddly. Below this size, its easier to use a Packed Column; where the packing can be random (eg just dumped in there and given a shake), or carefully positioned & stacked . For any particular type of packing, we can estimate how much of it is required to make one of these "ideal plates". See http://www.5continentsusa.com/cer-pack.htm for examples of different commercially available types of packing. These commercial packings are quite difficult to source, then expensive to purchase. They're designed for an industrial operation, where they're expected to be run continuously 24/7 for weeks or months at a time without fouling up. For a hobby distiller it is far easier, and with higher performance (%purity), to use common pot scourers (non-rusting stainless steel or copper) instead for packing, as we'll be cleaning them frequently (like after every 20L run).
Jim adds:
While gathering materials for my (first) reflux still, I came across an interesting material used for making batteries. It's a fine-mesh expanded metal made from copper by the Exmet Corporation. They make expanded metal from a variety of metals besides copper in sheets varying in width from .099 in. to 60 in. Their spec sheet is found at: http://www.exmet-corp.com/chart.html I don't know how you would calculate the void to surface ratio to get optimum results. I leave that to the "experts".One could roll a 30" wide sheet, for example, into a single piece that could be inserted into the column. It would have a very consistent internal structure. It would be easy to remove and clean.
Phil suggests a cheap supply of ceramic packings though ...
Have you checked the aquarium shops for ceramic rings used for pre filtration. I recently bought 2 litres for change from £10 (£4.85/l). I suspect they would do the same job. They also come in hex or tube shapes. There are also similar rings for bio filtration that have an open surface area so would perhaps be more efficient, though could be a bugger to clean
More about using plates (rather than packed columns ...
Ken : The idea with a fractionating column is a temperature gradient (falling of course) as you go UP the column, but a constant temperature ACROSS the column at any height. As the diameter of the column increases, it gets harder to achieve the constant temperature at a given height if you continue to use a packing material -- hence the plates. Plates give you resistance to flow in an upwards direction, but very easy "spreading" horizontally.
George : From what I have read, a general rule of thumb is; up to 4" you would be better off with scrubbers, anything bigger than 4" and scrubber will tend to channel, from 4" up to 8" you would do well with packing like pall rings and the like, from 10" and up you would do well to use plates. However I have seen plans for a 4" still with bubble cap plates and they claim to be the most efficient still made. Their claim not mine. To make the 10" bubble cap plate type still work you would need somewhere around a 10 hp boiler or around 350,000 btu input. It would also produce up to or around 30 gallon of ethanol per hou using a 10% wash. I have gleaned this information from a lot of different sources and complyed it myself, none of it is to be considered absolute. Their has been some writings about using some sort of perforated plate with the packing on the 6 to 8 inch stills to help even out the vapor flow.
On spacing I read once, and do not remember where that the spacing should be double the diameter as a rule of thumb. But the heat input, the quanity output, and the wash percent all effect this so it is hard to say. Their is no set rule to follow. Perforated plates require a lot of drilling and the bubble caps are hard to construct. Anything over a 6 to 8 inch would require quite the effort to bring up to speed. Unless you have a cheap source of heat, a motor of some type that runs constancely , the expense of bringing one of the bigger one up to steam would be very high.
One other thing that effects the plate type stills is wheather or not you are going to filter out the solids in your wash. If you are not then your plates would have to be designed to be self cleaning. If you do then the solids need to be compressed to get as much alcohol recovered as possible.
Gaw : Using the photos ... of a bubble type plate still in Holland I built a four inch eight plate still which seems to work quite well on top of a six gallon electric water heater with benefit of a thermostat which I added. To further the experiment of continuous distilling I added a thirty gallon pot with a connection three plates above the smaller unit and after the complete system reaches operating temps the unit seems to function quite well at 94-95 per cent. I used ss plates which I found in a salvage yard and soldered the bubblers into separate units which I then bolted together, believe it or not, with ss bolts and neoprene gaskets on each end of the four inch pieces.
Hennie writes:
I think the best solution for an ethanol distillation would be a packing of copper rings. These should not be too difficult to manufacture. Winding a copperwire on a thin rod with an electric drill and cutting the created spring to rings shouldn't be too difficult.