Distillation Theory
Summary of Distillation Theory
When you heat up a mixture of liquids, the more volatile components will tend to come off first. There is a bit of overlap (so it is never pure), but generally we can separate the ethanol from the water and other impurities present. The more alcohol in the liquid, the more alcohol will be in the vapor, so multiple distillations allow us to increase the strength & purity right up to 97.2%
The concept of [distillation] is really quite simple.
Mike Nixon has compiled an excellent pdf "Distillation - How it Works" explaining all this a lot clearer than what i have below. Right down to plate theory for heat exchangers, without making it complicated. Either e-mail Mike for it, or download it from me here (95 Kb, 16 Nov 99). A more detailed explanation is in his new book "The Compleat Distiller" at http://www.amphora-society.com.
Another explanation, geared towards distilling ethanol from agricultural products, is Purdue University's [1] note "Alcohol distillation : basic principles, equipment, performance relationships, and safety".
The University of Akron has a slide show covering the basics too. See also : "The Brewery's" Technical Library for articles on brewing related topics (extremely comprehensive) and AllTech's company homepage has much good literature.
Here's a somewhat simplified explanation ....(thanks to Mike)
When you have a mixture of liquids, each with its own boiling point when pure, then the boiling point of the mix will lie somewhere in the middle, and this will depend on the relative concentrations of each liquid. Pure water boils at 100 °C, and pure ethanol boils at 78.5 °C, but a mixture of water and ethanol will boil at some point in between. The major point about distillation is that when a mixture like that boils, then the vapor given off is richer in the most volatile component, and when that vapor condenses then the resulting liquid has a lower boiling point than the mix it came from. By repeating this boiling and recondensation process up a column, using packing to hold the condensed liquid at each stage, you can separate the components more and more.
So if you have a mixture of liquids each with a different boiling point, then you heat the mixture, it will heat up until the new intermediate boiling point is reached. When you first start a distilling run, the packing in the column will be at room temperature, so vapor given off by the boiler condenses on the first cool packing it reaches. In condensing, the vapor gives up a lot of heat, and this warms that packing until the liquid on it boils again. However, this liquid is richer in volatiles than the mix in the boiler, so its boiling point is lower. When it does boil again, from the heat given off by more condensing vapor, what you get is even richer in those most volatile components. This process of boiling and condensing continues up the column and, because the condensed liquid is always getting richer in volatiles, the temperature gradually falls the higher you go. The temperature at any point is governed solely by the boiling point of that liquid mix, and any attempt to interfere with that process will disrupt the separation that Nature is carrying out automatically.
In contrast, the boiling point of the mix left in the boiler will very slowly start to rise as it is left with less and less of the most volatile components.
If you started with a mixture (fermented wash) that is mostly water & ethanol, with trace amounts of methanol, propanol, etc. then the net result will be that the most volatile components will tend to rise in greater quantity up the column than their less volatile cousins, and will be found in greatest concentration at the top. This would mean that methanol, the most volatile of the lot, will win the race and you will able to collect it and set it aside. This continues until you have collected all of the "heads" (components that are more volatile than ethanol), and you can then collect just ethanol with a trace of water. You cannot get rid of that small amount of water, as once you reach a mix of 97.2% ethanol/water, with a boiling point of 78.2 °C, then you have reached a stable mix that no amount of re-boiling and re-condensation can change (at normal atmospheric pressure).
Once you have collected the main bulk of ethanol, then the components that are less volatile than ethanol, such as propanol and the bigger organic molecules, will start to reach the top, and you will have arrived at the stage called the "tails". These "tails" may be recycled in the next batch you do, for they still contain a lot of ethanol, or a proportion may be retained as they contain many of the compounds that give a spirit a distinctive flavor, like [whiskey] or [rum].
Note that you are not changing any part of your original brew - you're not "making" the alcohol, or converting it to something else or nasty. All you are doing is concentrating off the original brew into its various parts. There is no more methanol after you finish than what you started with. What does happen though, is that because most of the methanol comes off at once (first up), it is highly concentrated, and can damage you. You definitely don't want to be sampling the first portion of distillate that you collect. But once you have thrown away this part, you have guaranteed that the remaining distillate is safe enough to partake of.
Distillate Strength
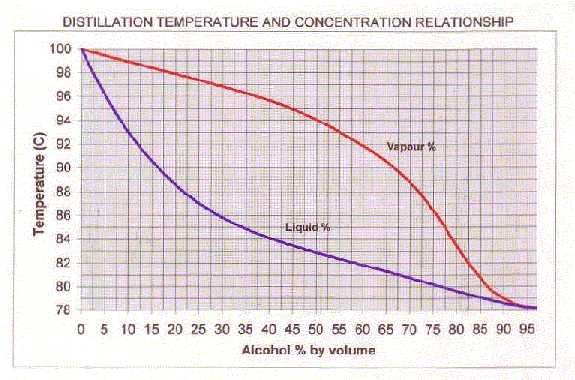
You can use the graph below (thanks to Grant D) to relate a liquid's % alcohol and its boiling point. It also shows the % alcohol of the condensed vapor. (The data for the graph (and heaps of other stuff) is on my Calculation page if you're interested in drawing it yourself.)
For example: A liquid of 10% alcohol will boil at 93 degrees (i.e. the point on the blue line directly above 10 on the [[Alc by Volume]|ABV] axis). If you head horizontally from the 93 degree point until you hit the red line, then drop down to the alcohol axis, it strikes 55%.
So, for a simple still, a 10% alcohol wash will boil at (initially) 93 degrees and the vapor, once condensed, will contain 55% alcohol by volume. Likewise, redistilling a 40% spirit should result in a brew around 80%.
Alcohol liquid - vapor curves
At % alcohol (liquid) the vapor will be % alcohol
Note that only 97.2% ethanol can be obtained by regular distillation of alcohol & water. Absolute ethanol (100%) is made by distilling with benzene which is poisonous. An azeotrope mixture of benzene, alcohol & water distills at 149F (65C) and removes the last few percent of water by vacuum distillation or by chemical means, e.g. using drying agents like molecular sieves with holes of 3 Angstrom or three ten billionths or a meter. This separates water which has a diameter of 2.5 Angstrom from ethanol which has a diameter of 4.5 Angstroms. Update: Phil advises me that most major commercial distilleries in Australia use Cyclohexane rather than Benzene.
Reflux Still Design
To increase the purity of the alcohol, and hence reduce the amount of "off-flavours" in it, you need to use a taller column, packed with something which has a large surface area (scrubbers are best), and have some of the vapour condensing and being returned back down over the packing as a liquid (reflux).
For a certain height of packing (called the HETP), the purity will improve - roughly 1x = approx 85% purity, 3x = 90%, 5x = 93%, and 7-9xfor 95%+. Just make it as high as what you want pure. For scrubbers the HETP is about 10cm (4 inches), whereas it is 24-38 cm (10-16 inches) for raschig rings or marbles.
See my Equations used during Reflux Distillation Calculations if you want to get into the detail of this.
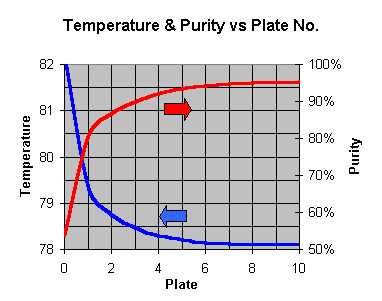
Purity is improved during distillation by allowing the rising vapor to mingle with some liquid at a slightly cooler temperature. In doing so, some of the water rich vapor will condense, supplying a bit of energy to allow some alcohol rich vapor to form from the liquid , and join the existing vapor. Each time this "mingling" is sufficient to reach equilibrium, the purity takes a "step" on the graph below:
Purity: Ethanol/Water Distillation Equalibrium Demonstration
Thanks to Chris Noonan for helping do this Applet.
This graph is of the "ethanol-water equilibrium" eg a liquid of 15% alcohol will be in equilibrium with a vapor at 65% alcohol. If this 65% vapor is then cooled to form a liquid (it will remain at 65%), the new liquid would then be at equilibrium with a 84% vapor, and so on. If you have a pot still, just set the plates to one.
You can see that due to the shape of the curve, most of the gains are early on; to get to the really high % purity, you need to take lots of steps later on. There is no way around this. If you want high purity, you have to work hard for it. Also note (particularly for inefficient columns with the equivalent of only 1-2 plates) that the starting % can also affect the final % achieved - hence a good idea to use the better yeasts.
Each of these "steps" represents an "ideal plate" where enough mingling of liquid & vapor allows them to come to equilibrium. If you don't allow enough mingling (equilibrium), then you won't achieve a full step, but end up a little shy of the target. You get the first step free - its the boiler/pot. Basically, off a 10% wash
1 = 53%
2 = 80%
3 = 87%
4 = 90%
5 = 92%
6 = 92.6%
7 = 93.3%
8 = 93.8%
9 = 94.2%
10 = 94.4%
One way of doing these steps is to do many single distillations, collect the vapour that comes off, condense it, clean out the still, and run it through the still again. This why pot stillers do double & triple distillations to get into the 80+ % range. But a Reflux column allows this to happen continuously; if given enough surface area to equalibriate on, the vapor can have gone through multiple distillations by the time it gets to the top of the column.
For each plate to work, it has to be at a particular temperature, slightly cooler than the one below, and warmer than the one above. Only then will it achieve its equilibrium and an increase in the alcohol purity. The differences are really fine too — its all happening only between 78.1 C and 82.2 C — quite a tight band to walk between.
Mike Nixon explains in a bit more detail ...
The process of separation depends on two facts:
1) when a vapor condenses then the resulting liquid has the same composition as the vapor, and the temperature at which this occurs is the same as the boiling point of that mixture. The boiling point lowers as the proportion of volatiles increases, so the temperature as you go up a column naturally decreases. One sticking point is that many think that a vapor only condenses when it encounters a surface that is cooler than the boiling point, but this is not so. Condensation occurs when there is a path for the latent heat of vaporization/condensation to be removed from the vapor, and the resulting liquid will remain at its boiling point if no further heat is removed.
2) when this liquid re-evaporates then the resulting vapor is richer in the most volatile components.
The packing is there simply to hold intermediate distillate in place so it can be bathed in hot, rising vapor and allow this second process to occur. As volatiles are further extracted from the intermediate distillate, the boiling point of what remains increases and the depleted liquid builds up, eventually dripping down the packing to a hotter level where it can again be stripped of more volatiles.
A cooling tube placed near the bottom of a column simply interrupts this natural progression and serves no useful purpose in the separation process. In contrast, the top cooling tube IS useful as it helps to return some of the vapor arriving at the top of the column to the packing, where it has a further chance of being stripped more thoroughly. This is what a condenser placed on top of a compound column does, but with more efficiency.
Temp and Purity Vs Number of Plates if Column Stills
This is where the various designs that have cooling tubes running through their columns at all different heights (eg Labmaster) come adrift — they don't allow the required sequence of temperatures to develop fully, and thus won't work at their full potential. They also don't allow all the refluxed liquid to do its job over the packing — the less liquid/vapor contact the poorer the "polishing" of the vapor will be.