Methanol: Difference between revisions
Uncle Jesse (talk | contribs) No edit summary |
Uncle Jesse (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
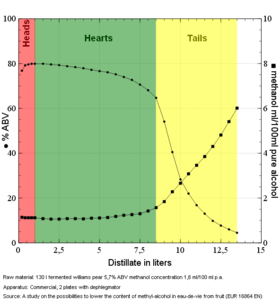
[[File:Methanol_concentration.png|thumb|280px|right|alt=Methanol concentration in an eau de vie run|Methanol concentration in an eau de vie run]] | |||
From [https://www.merriam-webster.com/ Webster]: | From [https://www.merriam-webster.com/ Webster]: | ||
A light volatile flammable poisonous liquid [[alcohol]] CH<sub>3</sub>OH used especially as a [[solvent]], antifreeze, or [[Denature|denaturant]] for [[Ethanol|ethyl alcohol]] and in the synthesis of other chemicals. | A light volatile flammable poisonous liquid [[alcohol]] CH<sub>3</sub>OH used especially as a [[solvent]], antifreeze, or [[Denature|denaturant]] for [[Ethanol|ethyl alcohol]] and in the synthesis of other chemicals. | ||
[[ | Methanol is associated with wood alcohol, and is not produced in any harmful quantity in a standard [[grain]] or [[fruit]] [[mash]]. However, it is produced in small quantities. For many years the community at [https://homedistiller.org/forum/ homedistiller] believed that methanol was mostly concentrated in the [[heads]]. However, [http://homedistiller.org/forum/viewtopic.php?f=33&t=40606 studies have shown] that methanol actually comes into play during the [[tails]]. See the graph of an [[eau de vie]] [[distillation]] [[run]]. | ||
Once ingested, methanol is metabolized into formic acid via formaldehyde in the liver. Formic acid causes damage to the optical nerves, resulting in blindness. | Once ingested, methanol is metabolized into formic acid via formaldehyde in the liver. Formic acid causes damage to the optical nerves, resulting in blindness. | ||
The amount of methanol produced is not enough to cause harm. Add to this the fact that the majority of the tails are not used in a final [[spirit]], and one can determine that a properly made [[ethanol]] spirit will not have harmful levels of methanol. | The amount of methanol produced is not enough to cause harm. Add to this the fact that the majority of the tails are not used in a final [[spirit]], and one can determine that a properly made [[ethanol]] spirit will not have harmful levels of methanol. | ||
Revision as of 00:55, 21 September 2017
From Webster:
A light volatile flammable poisonous liquid alcohol CH3OH used especially as a solvent, antifreeze, or denaturant for ethyl alcohol and in the synthesis of other chemicals.
Methanol is associated with wood alcohol, and is not produced in any harmful quantity in a standard grain or fruit mash. However, it is produced in small quantities. For many years the community at homedistiller believed that methanol was mostly concentrated in the heads. However, studies have shown that methanol actually comes into play during the tails. See the graph of an eau de vie distillation run.
Once ingested, methanol is metabolized into formic acid via formaldehyde in the liver. Formic acid causes damage to the optical nerves, resulting in blindness.
The amount of methanol produced is not enough to cause harm. Add to this the fact that the majority of the tails are not used in a final spirit, and one can determine that a properly made ethanol spirit will not have harmful levels of methanol.
External Links
A study on the possibilities to lower the content of methyl-alcohol in eaux-de-vie de fruits.